Extraction of Limonene Using Liquid Carbon Dioxide
by
I’d heard about a mythical method for extracting limonene from orange peel using supercritical carbon dioxide. Perhaps that doesn’t sound that exciting, but the fact that a friend of mine had seen this being done in a plastic tube, in an exhibition hall at a science education conference really got me thinking. How on earth can you get liquid carbon dioxide in a plastic tube? Surely the pressure would cause the tube to explode?
As a curious chemist, I just had to figure out how to do this and give it a go! Furthermore, 16-18 year-olds studying chemistry in UK schools need to know about the use of supercritical CO2 as a solvent in industrial processes as part of the Green Chemistry topic, and my teacher friends would welcome a video showing exactly how this might be done.
After extensive digging around on the net, I eventually found a procedure in an article in the journal Green Chemistry (McKenzie et al., 2004) which appeared to match the protocol described by my friend. Before trying out the experiment, I needed to find a centrifuge tube like those mentioned in the paper. A few phone calls to the usual suppliers indicated that I would need to invest a few hundred pounds as they were only available in packs of 1000! I then serendipitously discovered that a postgraduate student in the lab next to my office had a stash of the very same tubes and I gratefully left with a handful of them, before heading off to the local supermarket to buy an orange. Once again, I was forced to buy in bulk as they were only available in packs of 8, but at least I didn’t have to break the bank for that particular purchase…
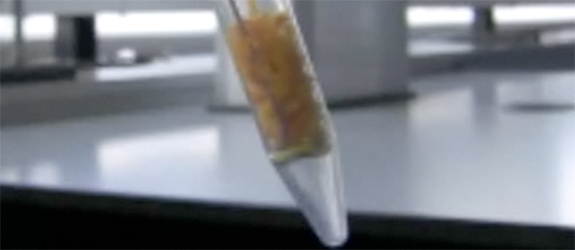
The first time I tried the experiment, I used 2 safety screens and wore a full-face visor/mask. I wasn’t taking any chances… After screwing the lid onto the tube, having crammed crushed dry ice onto the layer of orange peel, I took a step back behind the safety screens and covered my ears in readiness for the inevitable explosion. However, all my fears were allayed when the liquid carbon dioxide appeared in the tube and began to percolate through the orange peel. Suffice to say, the experiment is quite safe and does indeed work well, although I had to try it out several times before achieving the result observed in this video. Do please take care if you decide to do this yourself – read the Green Chemistry paper and take all due precautions. Chemistry should always be treated with respect!
References:
McKenzie, L. C.;Thompson, J. E.; Sullivan, R.; Hutchison, J. E., Green Chem., 2004, 6, 355


Ross
wrote on June 22, 2010 at 4:11 pm
It seems like the the CO2 was liquid and not supercritical. According to the phase charts you would need to have 30 C and 100 bar to be supercritical. Can you please comment on this?