Tearing It Up: Glycogen and Its Chemical and Biochemical Breakdown
by
 Not long ago, I was tasked with assaying for levels of glycogen in animal tissue. In most labs nowadays, glycogen is assayed by enzymatically hydrolyzing glycogen down to its component glucose monomers, and then enzymatically oxidizing that glucose in turn, producing hydrogen peroxide which can then react with a number of chromogens to produce a colored product.
Not long ago, I was tasked with assaying for levels of glycogen in animal tissue. In most labs nowadays, glycogen is assayed by enzymatically hydrolyzing glycogen down to its component glucose monomers, and then enzymatically oxidizing that glucose in turn, producing hydrogen peroxide which can then react with a number of chromogens to produce a colored product.
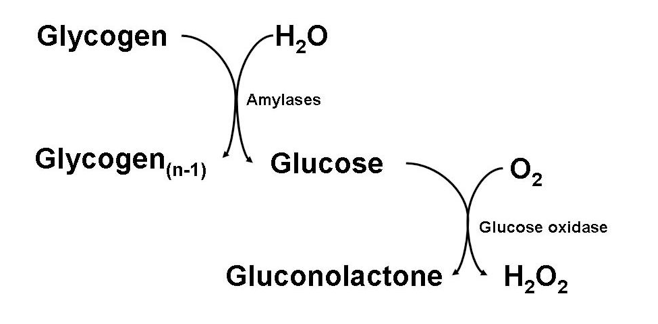
The problem with assaying glycogen in this manner, however, is that any baseline glucose present in your tissue extract will also be oxidized, produce hydrogen peroxide, and react with your chromogen. This can cause quite a high and significant blank, especially when your tissue handling and storage may not be as quick as it should, and some glycogen hydrolysis occurs in tissues post-collection.
A very good practice of avoiding this glucose blank, even within tissues that have been expertly handled, is to homogenize and process tissue samples in such a way that polymerized glycogen is precipitated, whereas monomeric glucose remains in solution. After the appropriated centrifugations and washes, glycogen is re-dissolved, now free of any interfering glucose. This method is to digest tissue samples in a strongly alkaline solution at high temperature: 30% potassium hydroxide (KOH), 95°C, 30 minutes (or more!) seems to be the norm. Needless to say, boiling your tissue samples in caustic solution is not a very common thing to do. It therefore was somewhat expected when I was asked an important question: under these harsh conditions, what’s to stop glycogen itself from being hydrolyzed along with a plethora of other biomolecules present in animal tissue?
I knew as soon as the question was asked that glycogen couldn’t possibly be hydrolyzed- at least not to a significant extent. Why? Well, if it were, then this sort of thing wouldn’t be published in the literature countless times, over many years. Okay, but other than standing on the shoulders of these published giants- seriously, chemically speaking- why isn’t glycogen hydrolyzed under the very same conditions that are used to dispose of entire human bodies in oh so many murder mysteries? Got lye?
First of all, how is glycogen normally broken down? And by “normally” I mean its metabolism within living organisms?
Intracellular glycogen metabolism
The breakdown of glycogen within cells has always fascinated me. Normally, when I think of the product of many glucose monomers linked together, I expect glucose itself, as shown in the assay scheme above. Instead, the intracellular “hydrolysis” product of glycogen is glucose-1-phosphate. Where did this phosphate come from?
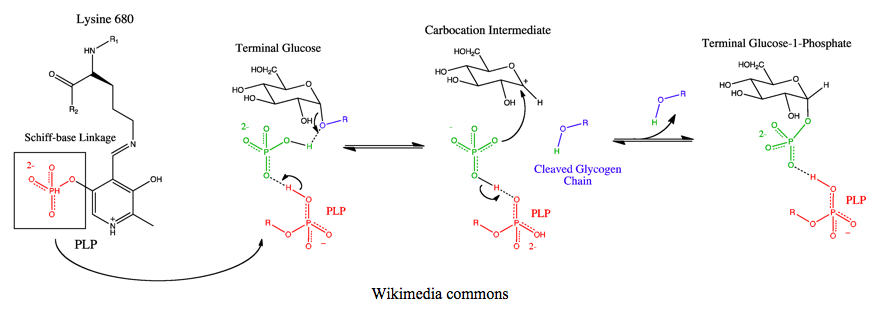
That’s because glycogen hydrolysis within cells isn’t a true hydrolysis. It’s a phosphorolysis. With the help of the pyridoxal phosphate (PLP) cofactor in its catalytic core, the glycosidic bond between two glucoses seemingly effortlessly collapses and scoops up a proton from inorganic phosphate, leaving behind glycogen reduced by one glucose monomer (glycogen(n-1)) and an oxonium glucose ion; a positively charged glucose carbocation stabilized by an electron lone pair from its neighboring oxygen. The remaining phosphate then extends a lone pair from any of its three deprotonated oxygens, bonding with the carbocation, creating the glucose-1-phosphate product. Glucose-1-phosphate is then converted to glucose-6-phosphate by phosphoglucomutase, allowing it to be shuttled into any number of the fates of glucose; dephosphorylation and export as free glucose, glycolysis, or oxidation in the pentose phosphate pathway.
Extracellular glycogen metabolism (digestion)
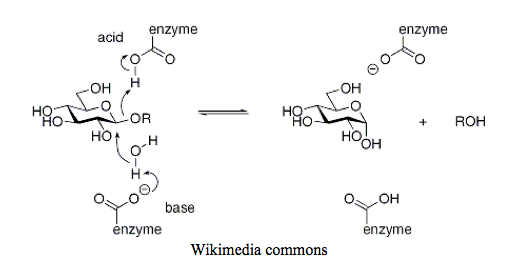
Outside of cells (such as within the digestive tract), glycogen is metabolized by a family of enzymes called the glycoside hydrolases, which include the amylase isozymes, as well as lyzosyme. This is actually quite a separate family of enzymes from glycogen phosphorylase; glycogen phosphorylase falls into the glycosyltransferases (EC 2.4) whereas glycoside hydrolases are within the sugar hydrolases (EC 3.2).
The mechanism by which glycogen hydrolysis occurs here has some similarities, and other differences. A cofactor like PLP is not involved, but rather the direct participation of amino acid side chains that are protonated and deprotonated. A deprotonated side chain, like aspartate or glutamate, abstracts a proton from water to generate a hydroxide nucleophile. This nucleophile then attacks the 1-carbon of the glycosidic bond, ousting the remaining glycogen(n-1), taking a proton from another amino acid side chain with it. All in one fluid step- at least, that’s how this figure shows it.
My favourite organic chemistry textbook (author’s note: it’s Organic Chemistry by Paula Yurkanis Bruice) depicts a slightly different catalytic mechanism for lysozyme, clearly showing two distinct steps with greater similarity to glycogen phosphorylase. The first is the collapse of the glycosidic bond and protonation of the remaining glycogen(n-1) polymer, as well as the formation of the oxonium ion on the glucose monomer. The second is the generation of hydroxide from water, and transfer of this hydroxide to the carbocation.
Chemical considerations
How does that scenic enzymatic route help us? We already know that enzymes do pretty extraordinary things at near-neutral pH that would otherwise require extreme conditions to achieve outside of a protein core. That tells us nothing new. Or does it?
The mechanism of glycogen phosphorylase, above, required the protonation of the electrons forming the glycosidic bond in order for glycogen to break away from a glucose monomer. The mechanism of the glycoside hydrolases- depending on which figure you look at and whether you believe it’s a 1-step or 2-step mechanism, either simultaneously or pre-emptively also requires protonation of the glycosidic bond.
Of course it does (I said after beating my head against a wall for several days)! In organic chemistry terms, glucose is an aldehyde in its linear form. Its cyclization is really the formation of a hemiacetal. Its linkage to another sugar to form a disaccharide (or longer) is the formation of an acetal. While the interconversion of the cyclic hexose hemiacetal is spontaneous, the full acetal needs something a bit more- something that we would expect from acetal equilibrium with any given organic molecule and not just glucose-glycoside acetals: acidic conditions.
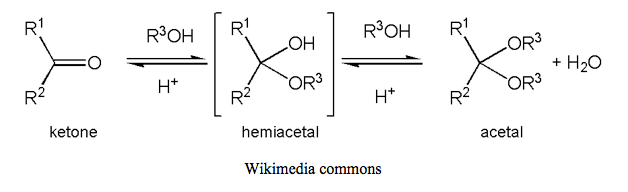
.
What biochemical polymers can be digested by alkaline hydrolysis?
- Triglycerides: the ester linkages between the central glycerol and each of the three bound fatty acids in a triglyceride molecule are hydrolyzed by hydroxide nucleophiles.
- Proteins: the amide bonds between amino acids are hydrolyzed. Several amino acids themselves are also destroyed under these conditions.
- Nucleic acids: RNA but not DNA is hydrolyzed in alkaline conditions. Why RNA and not DNA? The actual nucleophile that does the hydrolyzing is the 2’OH, which is generated by deprotonation from the alkaline conditions. This then results in an intramolecular hydrolysis of the phosphodiester linkages. No 2’OH, no hydrolysis.
.
Yet, glycogen remains intact under harsh alkaline conditions. When initially asked why that is, I was uncertain. It took looking through the catalytic mechanisms of two enzyme families to gain some insight as to why…
Ultimately, you know what was the biggest clue? 2nd year undergrad organic chemistry. Let it never be said that “we’ll never need this stuff again.”
.
.
.
Chris is originally from Montreal, and is a Comparative Biochemist and Physiologist. His educational and postdoctoral experiences have taken him from Montreal to Ottawa ON, State College PA, and finally back to Montreal’s biotech industry. In his spare time- as you would expect from a Canadian- Chris enjoys watching hockey and is a stalwart fan of the Montreal Canadiens and Ottawa Senators. You can keep up to date with the latest from Chris on Twitter.
.
.
.


pablo
wrote on May 10, 2013 at 8:46 am
hi Christopher,
very nice article!
but why glycogen remains intact under strong alkaline conditions?
cheers
pablo
Christopher Dieni
wrote on May 10, 2013 at 7:21 pm
Hi Pablo, thanks a lot. Essentially, from an organic chemistry standpoint, I think that the only way to create/break down hemiacetals and acetals is under acidic conditions. I don't believe that the anomeric carbon is enough of an electrophile to be attacked by hydroxide even under strong alkaline conditions. But as for why that is… honestly it might be worth talking to an organic chemist or a carbohydrate chemistry for a more complete answer!